


We perform initial formulation study, sample making and other processes at the research and development department,
in order to conduct pharmaceutical development according to the client’s request.
We also check the quality of the products, and conduct quality management / quality assurance tasks at the quality assurance
department to ensure that the drug manufacturing is under the control of GMP.
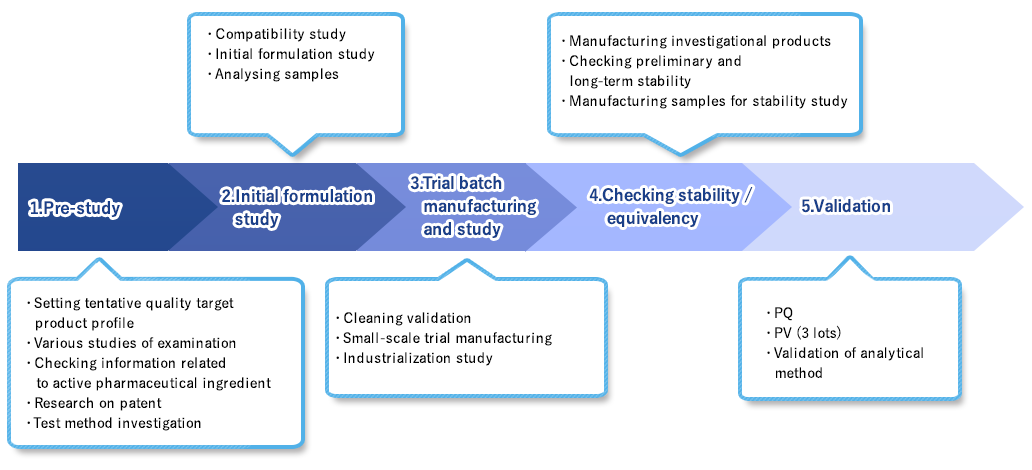
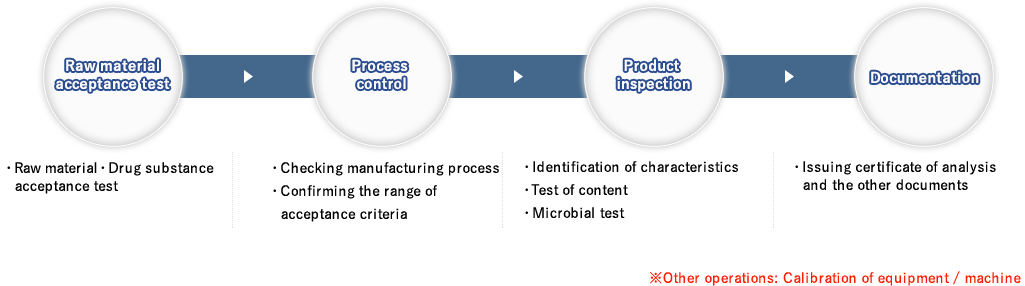
The manufacturing and development flow consists of 5 steps.
The flow starts off with (1) Setting tentative specification, then (2) Formulation study, (3) mall-scale trial manufacturing, (4) checking stability /
equivalency, and (5) validation. We ensure the safety and quality of the product in each process and work towards the safe and secure development of pharma film.
As the photo shown below, R&D team tests and evaluates to investigate the film in our lab.


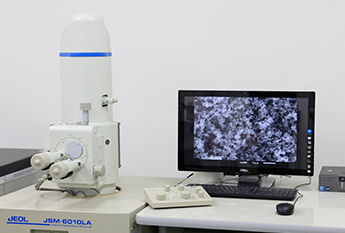
Scanning electron microscope

Contact thermometer Film thickness measurement

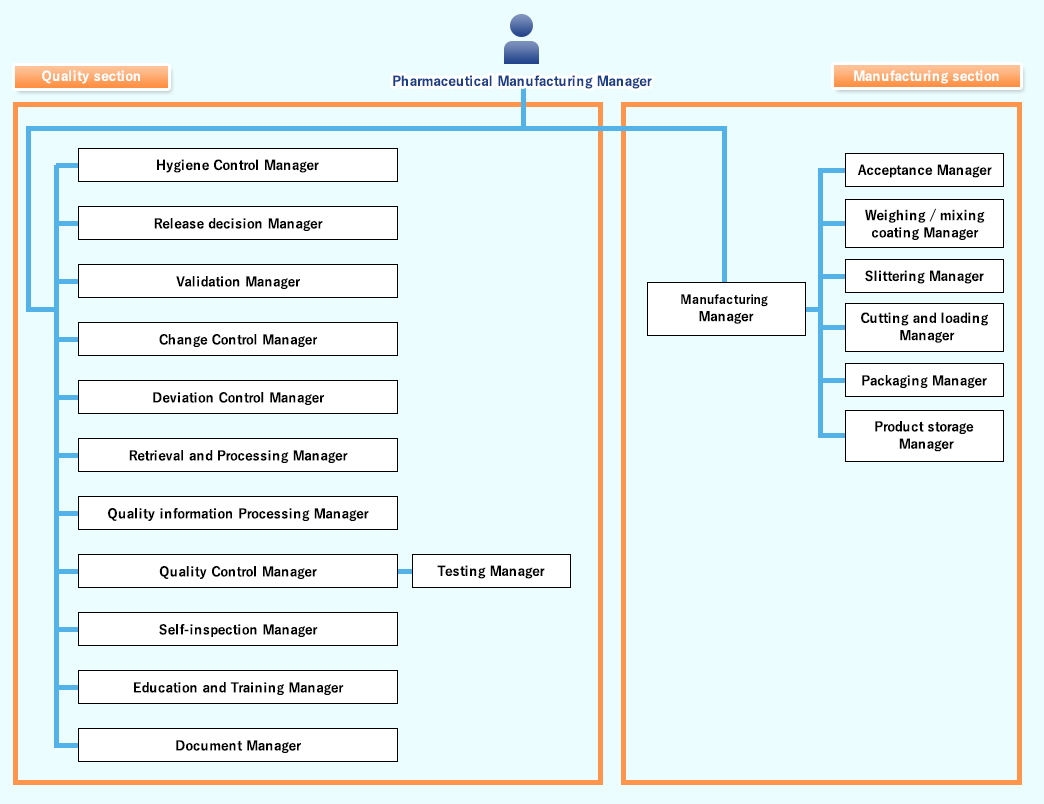
The supervisors in each department fulfills their respective roles Under the GMP management system related to pharmaceutical product manufacturing.

HPLC

Dissolution tester

Karl Fischer moisture meter